RECIST 1.1
With rapid technical innovations in imaging techniques including MDCT and PET/CT
There was a need for revision
Published in January 2009 by RECIST working group
Published to overcome the shortcoming of RECIST 1.0
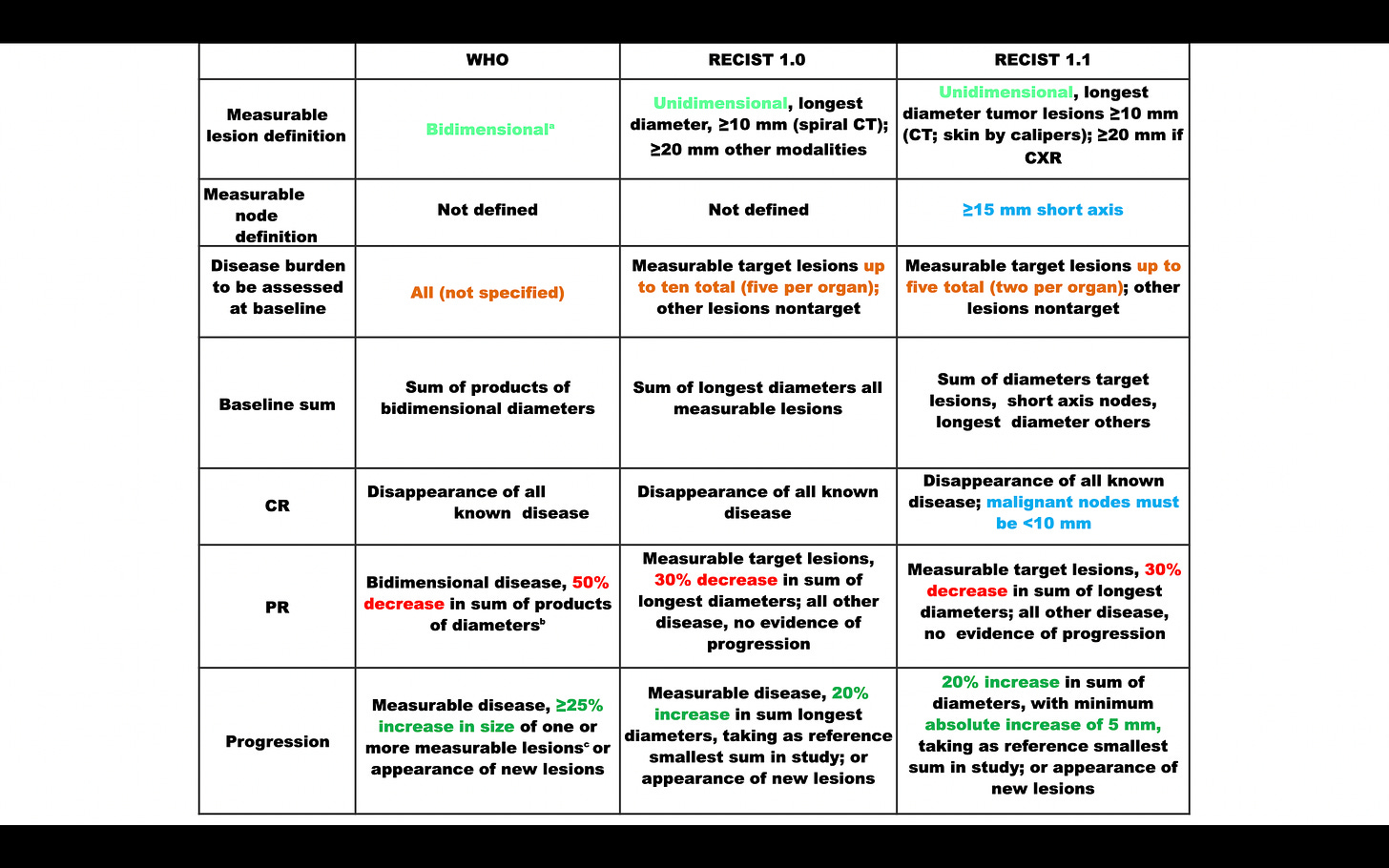
Major changes in RECIST 1.1
Number of target lesions;
Assessment of pathologic lymph nodes;
Clarification of disease progression;
Clarification of unequivocal progression of non-target lesions;
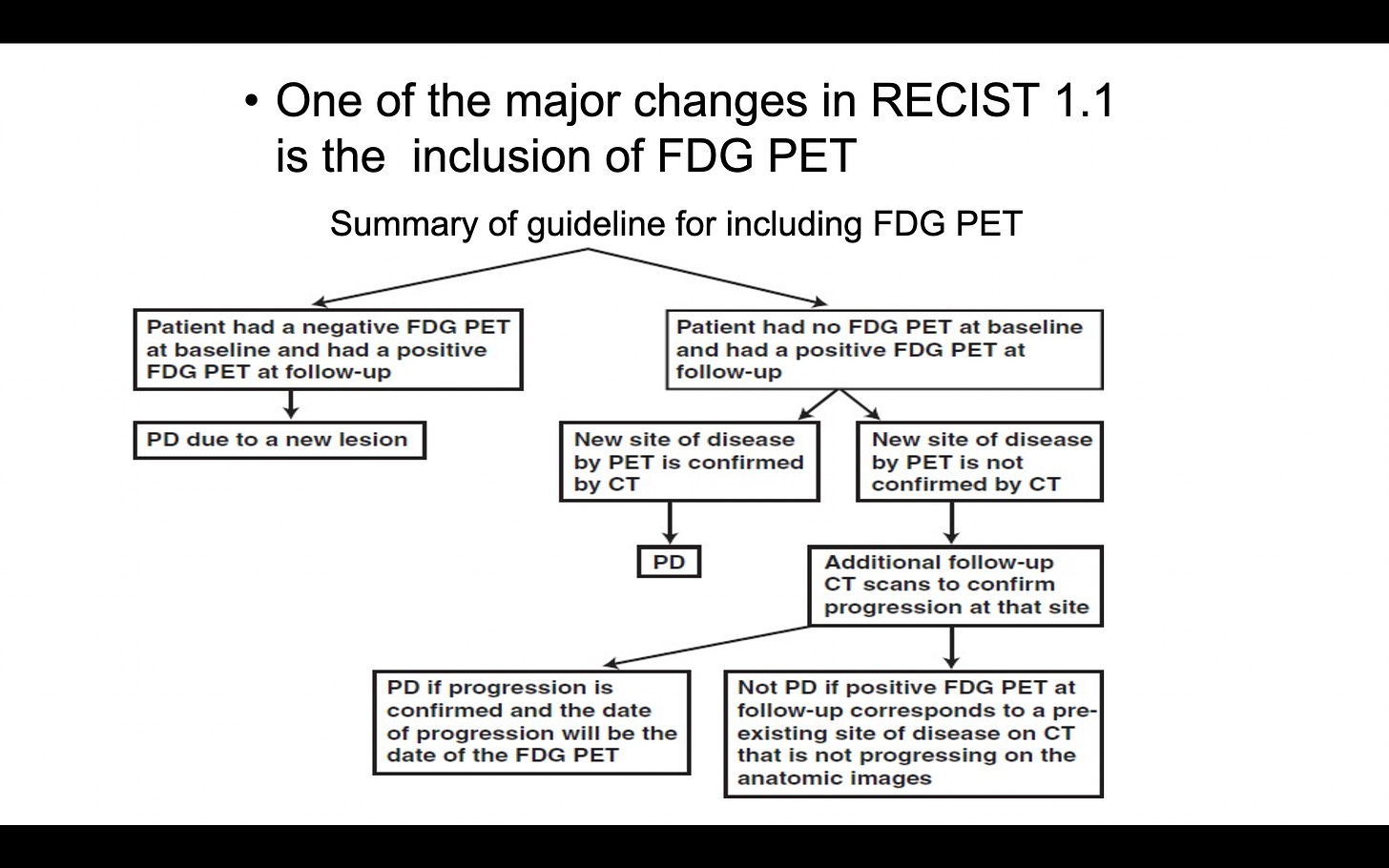
Inclusion of 18F-FDG PET in the detection of new lesions
No. of target lesions
Number of target lesions was reduced
from 5 per organ to 2 per organ and
from a maximum of 10 total to a maximum of 5.
assessment of five lesions per patient did not influence the overall response rate and only minimally affected progression-free survival.
Assessment of pathological lymph nodes
No guidelines in RECIST 1.0
Lymph nodes with a short axis of ≥ 15 mm are considered measurable.
as opposed to the longest axis used for other target lesions
Lymph nodes <10mm are defined as non-pathological
In-between size- non target lesion.
Clarification of disease progression
Clarification of Unequivocal Progression of Nontarget Lesions
SD or PR in target disease + substantial worsening in nontarget disease = PD
E.g. increase in pleural effusion from trace to massive
Increase in lymphangitis disease from localized to widespread.